An Improved Solution to Combat Heart Failure
“Whisper” – catheter based pump
“Freedom” – wireless powered chronic implant
Motor controller

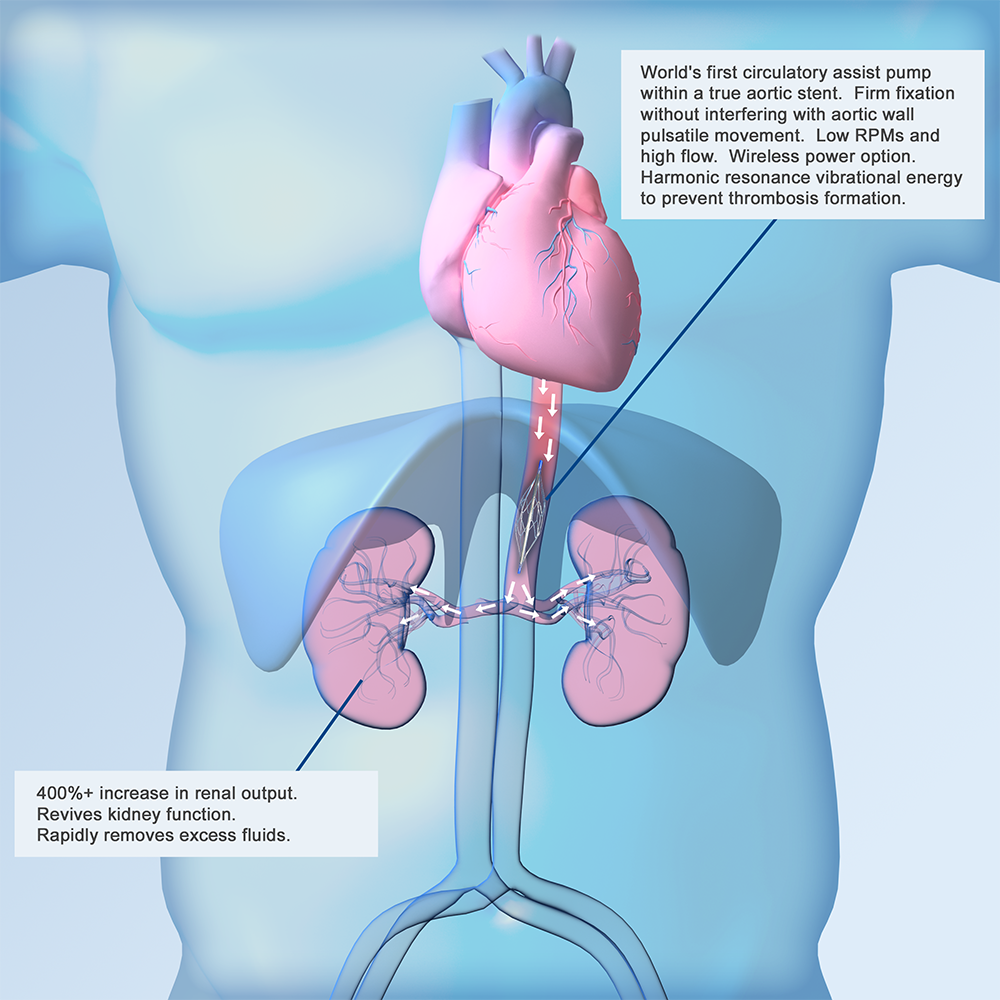
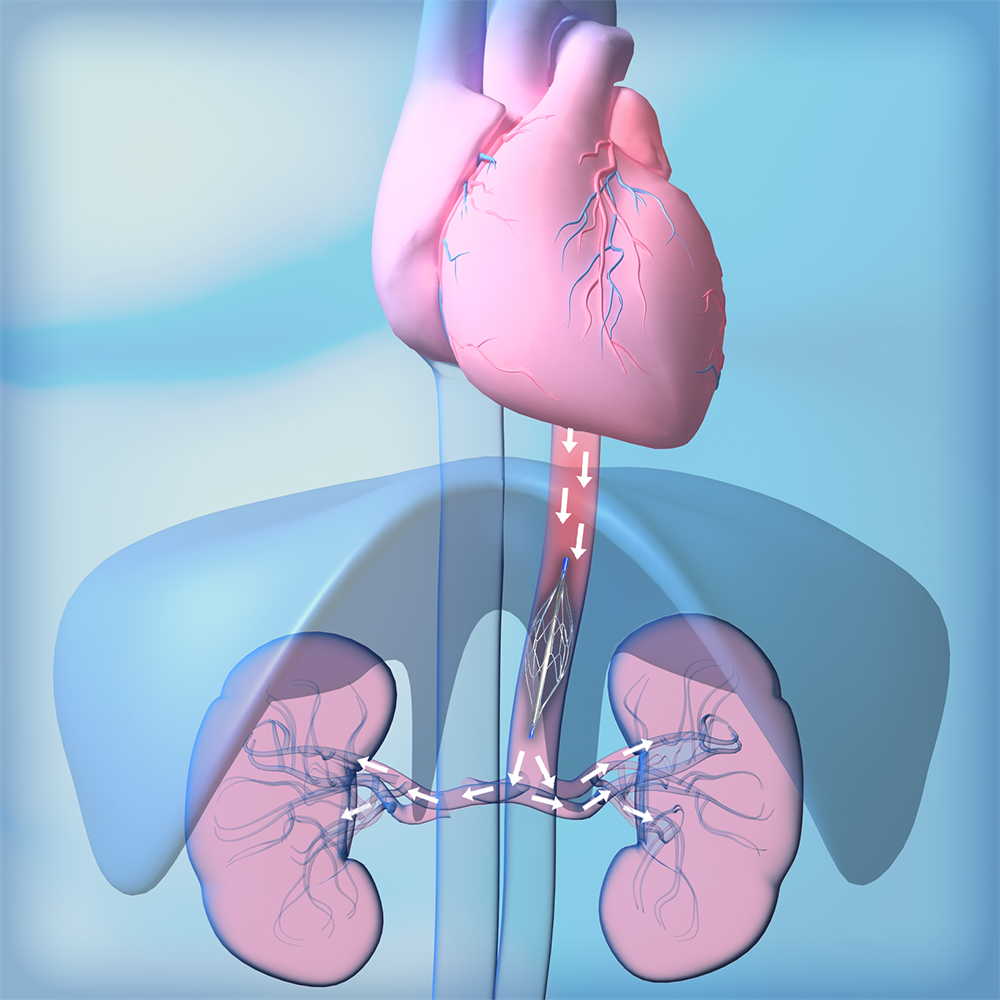
Latest News

CAUTION Disclaimer and Warning: Products described on this web site are in early stage development and are not yet proven safe or effective in statistically significant controlled clinical studies. Any statement or phrases implying efficacy or safety in any form are considered modified by “intended to” or “designed to”. Investigational use only in countries where investigation is permitted by law and proper filings have been made and appropriate regulatory clearances have been granted. Any use of the product(s) must be in an authorized clinical study with institutional review board (ethics committee) approval and proper patient consent procedures followed. For other countries product is only available for laboratory investigation by credentialed institutions and investigators with proper clearances with a research agreement in place with a study sponsor. NOT AVAILABLE FOR SALE.
